Radiopharmaceuticals are unique medicinal formulations that contain radioactive isotopes. They are mainly used in diagnosis, therapy, and research. Understanding their properties and handling is essential in pharmaceutical practice.
What are radiopharmaceuticals?
Radiopharmaceuticals are a unique class of drugs that contain radioactive isotopes (radioisotopes). They are used in nuclear medicine for both diagnostic and therapeutic purposes. Unlike conventional drugs that produce a pharmacological effect through chemical interactions, radiopharmaceuticals work by emitting radiation, which is then detected by special imaging equipment or used to destroy diseased cells.
Radioactivity
Radioactivity is the spontaneous process by which an unstable atomic nucleus loses energy by emitting radiation. This process, known as radioactive decay, transforms the unstable nucleus into a more stable one. The emitted radiation can take the form of particles or high-energy electromagnetic waves.
Types of Radioactive Decay
The three main types of radiation emitted during radioactive decay are:
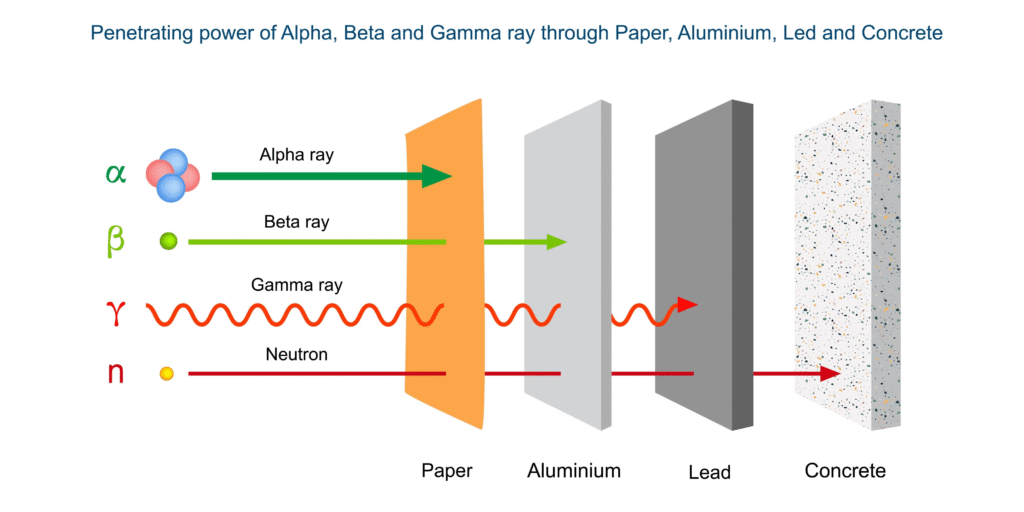
- Alpha Decay (α): An unstable nucleus emits an alpha particle, which consists of two protons and two neutrons (a helium nucleus). This type of decay reduces the atomic number by 2 and the mass number by 4. Alpha particles have a low penetrating power and can be stopped by a sheet of paper.
- Beta Decay (β): In beta decay, a neutron in the nucleus is converted into a proton, emitting a high-energy electron (beta particle) and an antineutrino. This increases the atomic number by 1, while the mass number remains the same. Beta particles have a medium penetrating power and can be stopped by a thin sheet of aluminum.
- Gamma Decay (γ): This is not a particle emission but the release of excess energy from an excited nucleus in the form of a high-energy electromagnetic wave (gamma ray). Gamma decay often accompanies alpha or beta decay. Gamma rays have a very high penetrating power and require thick lead or concrete to be shielded effectively.
Properties of Radiations
The properties of different types of radiation are determined by their composition, charge, and energy. This influences their ability to ionize atoms, penetrate matter, and cause biological damage. The primary types of radiation are alpha, beta, and gamma, but other particles like protons and neutrons are also used in medicine and research.
1. Alpha (α) Radiation
Alpha radiation consists of alpha particles, which are essentially helium nuclei composed of two protons and two neutrons.
2. Beta (β) Radiation
Beta radiation consists of beta particles, which are high-energy electrons or positrons.
3. Gamma (γ) Radiation
Gamma radiation is high-energy electromagnetic radiation, similar to X-rays but with a higher energy. It is not a particle.
Other Types of Radiation
- Neutron Radiation: This consists of neutrons, which are uncharged particles. Because they lack charge, they do not directly ionize atoms but can penetrate deep into matter. They interact with atomic nuclei, which can cause other forms of radiation (indirect ionization).
- Proton Radiation: This consists of protons, which are positively charged particles. They have a unique property called the Bragg peak, where they deposit most of their energy at a specific, targeted depth, with very little radiation dose beyond that point. This makes proton therapy highly effective for treating deep-seated tumors with minimal damage to surrounding healthy tissue.
- Positron Radiation: A type of beta decay, this involves the emission of a positron (an antielectron). Positrons are used in Positron Emission Tomography (PET) scans. When a positron collides with an electron, they annihilate each other, producing two gamma rays that are detected by the scanner.
FAQ – Radiopharmaceuticals
1. What are radiopharmaceuticals?
Radiopharmaceuticals are medicinal formulations that contain radioactive isotopes and are used for diagnosis or treatment of various diseases.
2. What is radioactivity?
Radioactivity is the spontaneous emission of radiation from unstable atomic nuclei in the form of alpha (α), beta (β), or gamma (γ) rays.
3. How is radioactivity measured?
It is measured using devices like Geiger-Müller counters, scintillation counters, or ionization chambers. Units of measurement include Becquerel (Bq) and Curie (Ci).
4. What are the properties of α, β, and γ radiations?
- Alpha (α): Heavy, positively charged, low penetration, stopped by paper or skin.
- Beta (β): Light, negatively or positively charged, medium penetration, stopped by aluminum sheet.
- Gamma (γ): No charge, high penetration, requires lead shielding.
5. What is the half-life of a radioactive isotope?
Half-life is the time required for half of the radioactive atoms in a sample to decay.
