Biological systems are powered and maintained by an extraordinary set of molecules that not only provide structure but also energy for life processes. Two of the most fundamental concepts in biochemistry are biomolecules—the building blocks of life—and bioenergetics, which explains how energy is generated and utilized in cells. Together, they form the basis of cellular function, metabolism, and pharmaceutical applications.

Introduction to Biomolecules
Biomolecules are complex organic molecules that are essential for life. They are the building blocks of living organisms and are involved in all of the biological processes that keep an organism alive, from metabolism and energy storage to genetic information and structural support.
Major Classes of Biomolecules
There are four major classes of biomolecules:
- Carbohydrates: These are the primary source of energy for most living organisms. They are made of carbon, hydrogen, and oxygen atoms and are classified based on the number of sugar units they contain.
- Monosaccharides: Simple sugars like glucose and fructose.
- Disaccharides: Two sugar units, like sucrose (table sugar).
- Polysaccharides: Long chains of sugars, like starch (for energy storage in plants) and cellulose (for structural support).
- Proteins: These are large, complex molecules that perform a vast range of functions in the body. They are polymers of amino acids.
- Enzymes: Proteins that act as catalysts for biochemical reactions.
- Structural proteins: Provide support for cells and tissues (e.g., collagen).
- Hormones: Chemical messengers (e.g., insulin).
- Lipids (Fats): A diverse group of non-polar molecules that are not soluble in water. They are essential for energy storage, cell membrane structure, and as signaling molecules.
- Fats and oils: Used for long-term energy storage.
- Phospholipids: The main component of cell membranes.
- Steroids: Hormones like cholesterol and testosterone.
- Nucleic Acids: These molecules carry the genetic information that directs the growth, development, and reproduction of all living organisms. They are polymers of nucleotides.
- DNA (Deoxyribonucleic Acid): Contains the genetic blueprint for an organism.
- RNA (Ribonucleic Acid): Involved in the synthesis of proteins.
Biomolecules are formed through polymerization, where smaller units called monomers link together to form long chains called polymers. For example, amino acids are the monomers that polymerize to form the polymer protein.
What is Bioenergetics?
Bioenergetics is the study of energy flow and transformation in living organisms. It’s a fundamental field of biochemistry that focuses on how cells acquire, convert, and use energy to perform biological work. The core principles of bioenergetics are based on the laws of thermodynamics, which govern all energy transfer.
Key Principles
- First Law of Thermodynamics: Energy cannot be created or destroyed, only converted from one form to another. In living systems, this means the chemical energy in food is converted into other forms of energy (e.g., kinetic energy for movement, thermal energy as heat).
- Second Law of Thermodynamics: In any energy conversion, some energy is lost as heat, leading to an increase in the entropy (disorder) of the universe. This is why metabolic processes are not 100% efficient.
Metabolic Processes
Bioenergetics is primarily concerned with the two major metabolic pathways that manage energy:
- Catabolism: The breakdown of complex molecules into simpler ones, releasing energy in the process. This is an exergonic reaction. For example, the catabolism of glucose through cellular respiration releases energy to be stored as ATP.
- Anabolism: The synthesis of complex molecules from simpler ones, which requires an input of energy. This is an endergonic reaction. For example, the synthesis of proteins from amino acids or the storage of glucose as glycogen.
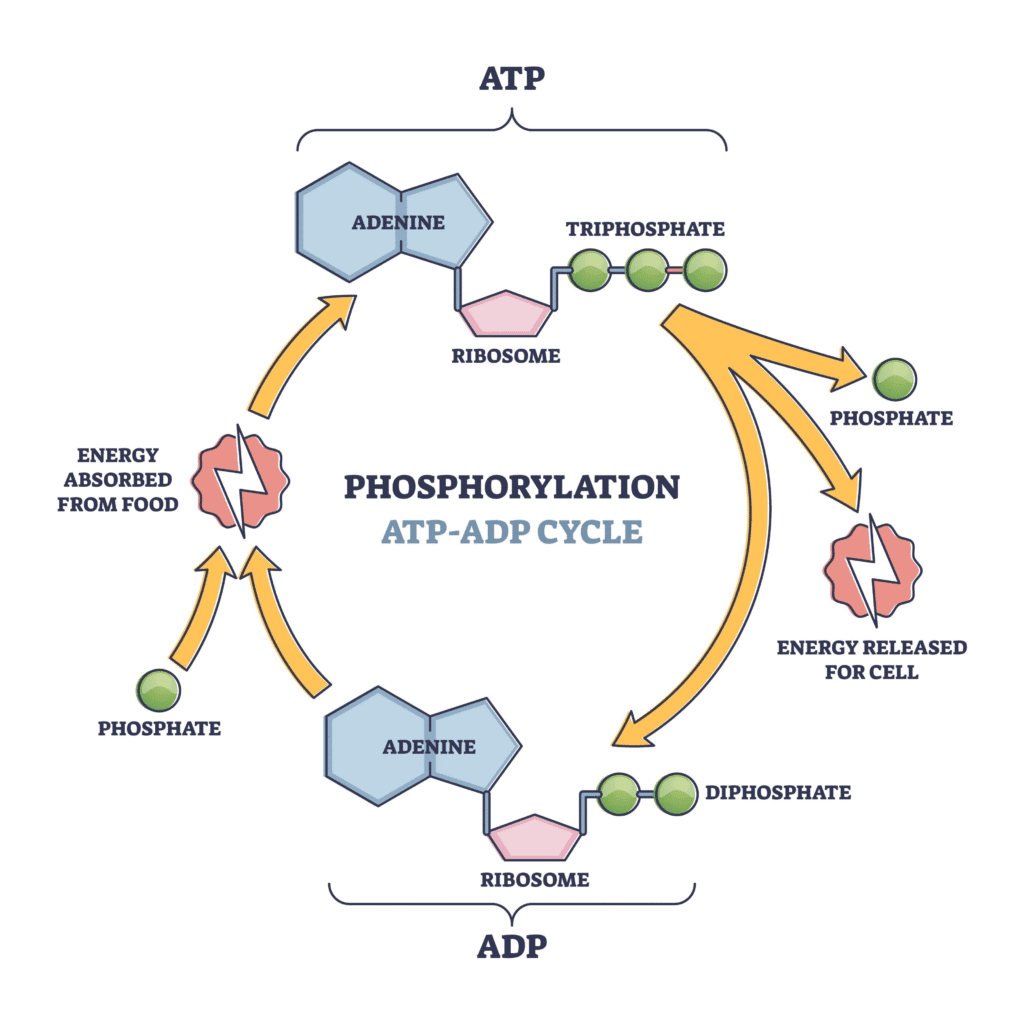
The Role of ATP ATP (adenosine triphosphate) is the central molecule in bioenergetics. It is often referred to as the “energy currency” of the cell. Energy released from catabolic reactions is captured and stored in the high-energy phosphate bonds of ATP. The hydrolysis of ATP to ADP and phosphate releases this stored energy to power anabolic reactions and other cellular activities.
