Gastrointestinal (GI) agents play a crucial role in treating various stomach and intestinal disorders. This unit introduces commonly used acidifiers, antacids, and cathartics, explaining their roles, ideal properties, and examples used in pharmacy.

Acidifiers
Acidifiers are inorganic or organic chemicals that produce or become acid in the body, lowering the pH of a specific fluid or area. They are used in pharmacy for various purposes, from correcting low stomach acidity to preventing certain types of kidney stones.
Types of Acidifiers
Acidifiers are categorized based on their therapeutic use and mechanism of action:
- Gastric Acidifiers: These are used to temporarily restore stomach acidity in patients with hypochlorhydria or achlorhydria, conditions where the stomach secretes deficient amounts of hydrochloric acid. A common example is dilute hydrochloric acid (HCl), which provides the necessary acidity for proper digestion of food.
- Urinary Acidifiers: These agents are used to lower the pH of urine. This can be beneficial for:
- Treating urinary tract infections by making the environment less hospitable to bacteria.
- Preventing the formation of certain kidney stones, particularly struvite stones, which form in alkaline urine.
- Examples include ammonium chloride (NH4Cl) and ascorbic acid (Vitamin C).
- Systemic Acidifiers: These are administered to lower the overall alkalinity of the body’s fluids. They are typically used to treat metabolic alkalosis, a condition where the blood pH is too high. Ammonium chloride is an example of a systemic acidifier, as the ammonium ion is converted to urea in the liver, leaving behind a hydrogen ion that lowers the body’s pH.
What are Antacids?
Antacids are a class of over-the-counter (OTC) medications used to neutralize excess stomach acid and relieve symptoms of heartburn, indigestion, and acid reflux. They are typically weak bases, or alkaline substances, that work directly in the stomach to counteract the strong acid produced there.
How Antacids Work
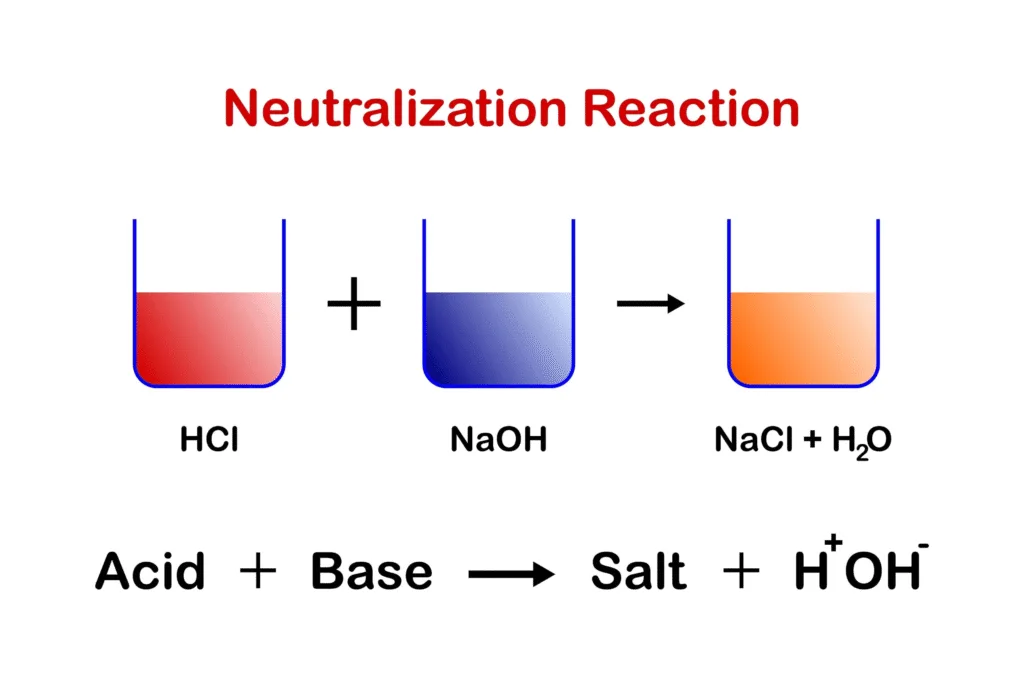
Antacids function through a simple acid-base neutralization reaction. The active ingredient in an antacid (a base) reacts with hydrochloric acid (HCl) in the stomach to produce a salt and water, thus raising the stomach’s pH and reducing its acidity. This provides rapid, but temporary, relief from discomfort.
For example, a common antacid, magnesium hydroxide, reacts with stomach acid as follows:
Mg(OH)2+2HCl→MgCl2+2H2O
Common Types of Antacids
Antacids are made from various inorganic salts. They are often used in combination to balance their side effects.
- Aluminum Hydroxide (Al(OH)3): This is a slowly-acting antacid that can cause constipation. It also binds to phosphate in the gut, which can be useful for patients with kidney disease but may lead to hypophosphatemia with long-term use.
- Magnesium Hydroxide (Mg(OH)2): Known as Milk of Magnesia, this is a fast-acting antacid that often has a laxative effect, which can cause diarrhea.
- Calcium Carbonate (CaCO3): A fast-acting and potent antacid that can also cause constipation. It’s also a source of dietary calcium, making it a popular choice, but excessive use can lead to hypercalcemia. A common brand name is Tums.
- Sodium Bicarbonate (NaHCO3): This antacid works very quickly but is also short-acting. It can produce carbon dioxide gas in the stomach, leading to bloating and belching. Due to its high sodium content, it’s generally not recommended for people on a sodium-restricted diet or with conditions like high blood pressure or heart failure.
Definition of Cathartics
Cathartics are a class of medications used to accelerate and promote defecation. They are stronger than typical laxatives, leading to a more complete and often more fluid evacuation of the bowel. Cathartics are also known as purgatives.
Mechanism of Action
Cathartics work through a few different mechanisms to increase bowel movements.
- Osmotic Action: These are known as saline cathartics. They are poorly absorbed salts or sugars that draw water into the intestines through osmosis. This increases the fluid content and bulk of the feces, which stimulates intestinal distention and promotes peristalsis (the wave-like muscle contractions that move waste through the digestive tract). Examples include magnesium sulfate (Epsom salt) and sodium phosphate.
- Stimulant Action: Also called stimulant laxatives, these drugs directly irritate the intestinal lining or stimulate the nerves in the intestinal wall. This causes an increase in intestinal motility and secretions, leading to a strong, propulsive bowel movement. Examples include senna and castor oil.
FAQs – Gastrointestinal Agents
1. What is the difference between acidifiers and antacids?
- Acidifiers increase stomach acidity to aid digestion.
- Antacids reduce excess acid to relieve symptoms like acidity or ulcers.
2. Why are combinations of antacids preferred?
Combining agents balances side effects like constipation (Aluminum) and diarrhea (Magnesium) for smoother action.
3. Can cathartics be used daily?
No. Prolonged use can lead to dependency and loss of normal bowel function.
4. Why is sodium bicarbonate not ideal for long-term antacid use?
It produces CO₂ gas, may cause bloating, and risks metabolic alkalosis if overused.
5. How are kaolin and bentonite different from other cathartics?
They are adsorbents, not true laxatives. They bind irritants in diarrhea rather than stimulate bowel movement.
