Enzymes are often called the molecular workhorses of life. They are biological catalysts that accelerate chemical reactions inside living organisms, making life processes possible at body temperature and normal physiological conditions. Without enzymes, reactions like digestion, energy production, and DNA replication would occur far too slowly to sustain life.
This unit explores the properties, classification, kinetics, regulation, and applications of enzymes, along with their coenzymes and inhibitors, which together form the foundation of enzymology.

Introduction to Enzymes
Enzymes are biological molecules, primarily proteins, that act as catalysts to speed up the rate of biochemical reactions without being consumed in the process. They are essential for virtually all metabolic processes in the body, from digestion to DNA replication.
Key Characteristics of Enzymes
- Catalysts: Enzymes increase the rate of a reaction by lowering the activation energy, which is the minimum energy required for a reaction to occur. They do this by providing an alternative reaction pathway.
- Specificity: Enzymes are highly specific to their substrates, much like a lock and key. A specific enzyme will typically only bind to a specific substrate or a small group of chemically similar substrates. The part of the enzyme that binds to the substrate is called the active site.
- Efficiency: Enzymes can increase reaction rates by millions or even billions of times. They can also be regulated to turn reactions on or off as needed.
- Reusability: Enzymes are not consumed during the reaction and can be used over and over again.
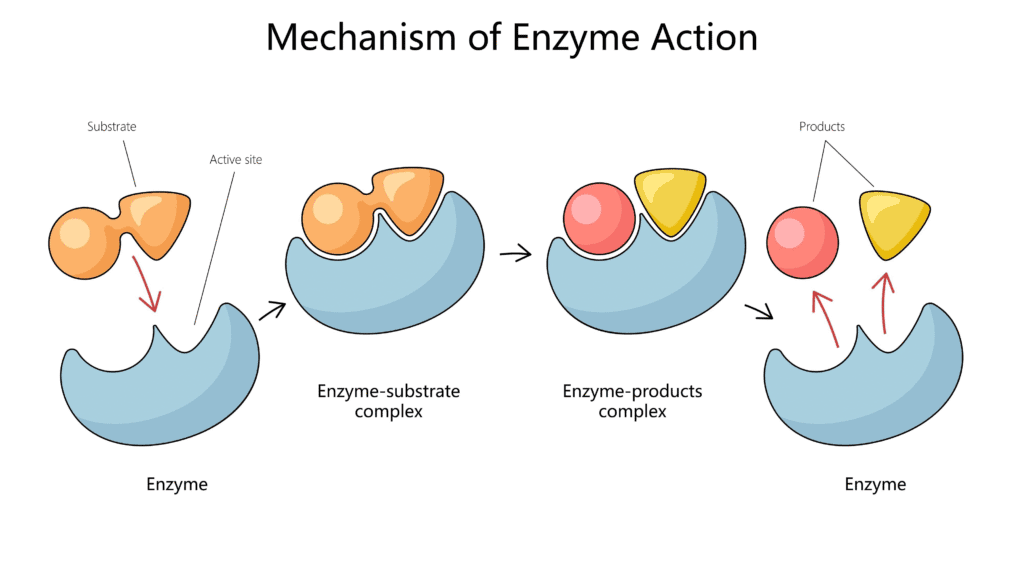
Mechanism of Action
The action of an enzyme involves a few steps:
- Substrate Binding: The substrate molecule binds to the enzyme’s active site, forming an enzyme-substrate complex. The active site’s shape is complementary to the substrate, ensuring specificity.
- Catalysis: The enzyme facilitates the reaction by putting stress on the chemical bonds of the substrate, orienting the molecules correctly, or by creating a more favorable environment.
- Product Release: Once the reaction is complete, the products are released from the active site, and the enzyme is free to bind to a new substrate.
Nomenclature and IUB Classification
Enzyme nomenclature is a system for naming enzymes based on the type of reaction they catalyze and the substrates they act on. The most widely accepted system is the IUBMB (International Union of Biochemistry and Molecular Biology) classification, which assigns a unique four-part number to each enzyme, known as the EC (Enzyme Commission) number.
IUBMB Classification System
The EC number consists of four numbers separated by periods (e.g., EC 2.7.1.1).
- First digit: The major enzyme class, indicating the type of reaction catalyzed.
- Second and third digits: Subclasses that provide more specific information about the reaction, such as the type of bond broken or the group transferred.
- Fourth digit: A serial number that uniquely identifies a specific enzyme within its sub-subclass.
There are seven major classes of enzymes, each with a unique first digit:
- Oxidoreductases (EC 1): Catalyze oxidation-reduction reactions (transfer of electrons or hydrogen atoms).
- Transferases (EC 2): Catalyze the transfer of a functional group from one molecule to another.
- Hydrolases (EC 3): Catalyze the hydrolysis of various bonds (breaking a bond by adding water).
- Lyases (EC 4): Catalyze the cleavage of bonds by means other than hydrolysis or oxidation, often resulting in the formation of a double bond.
- Isomerases (EC 5): Catalyze the rearrangement of atoms within a molecule to form an isomer.
- Ligases (EC 6): Catalyze the joining of two molecules, often coupled with the hydrolysis of ATP.
- Translocases (EC 7): Catalyze the movement of ions or molecules across membranes.
